Answer:
![K_2=([NOBr]^4_(eq))/([NO]^4_(eq)[Br]^2_(eq))](https://img.qammunity.org/2021/formulas/chemistry/college/d1jd0jxmzka3bs6uwo7a9khkcn11hbw34w.png)
Step-by-step explanation:
Hello,
In this case, for the equilibrium condition, the equilibrium constant is defined via the law of mass action, which states that the division between the concentrations of the products over the concentration of the reactants at equilibrium equals the equilibrium constant, for the given reaction:
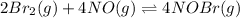
The suitable equilibrium constant turns out:
![K_2=([NOBr]^4_(eq))/([NO]^4_(eq)[Br]^2_(eq))](https://img.qammunity.org/2021/formulas/chemistry/college/d1jd0jxmzka3bs6uwo7a9khkcn11hbw34w.png)
Or in terms of the initial equilibrium constant:
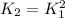
Since the second reaction is a doubled version of the first one.
Best regards.