Answer:
Wavelength of light in (nm) = 579 nm
Step-by-step explanation:
At first you find out the amount of energy needed to just eject one electron. This is given by
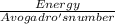
this energy is given in question in kj/mole. This
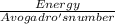 is the work function of cesium for each electron is equal to the planc'k einstein equation.
is the work function of cesium for each electron is equal to the planc'k einstein equation.