Answer:
1.
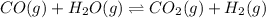 , remain the same.
, remain the same.
2.
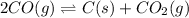 ,increase.
,increase.
3.
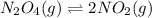 , decrease.
, decrease.
Step-by-step explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
Increase the pressure
If the pressure of the container is increased, the volume will decrease according to Boyle's Law. Now, according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease in pressure is taking place.
1.
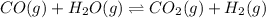
On increasing the pressure ,the equilibrium will shift in direction where decrease in pressure is taking place,so reaction will shift in no direction because number of gaseous moles are same on both sides. Hence no increase in number of moles of products.
2.
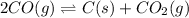
On increasing the pressure ,the equilibrium will shift in direction where decrease in pressure is taking place,so reaction will shift in forward direction because number of gaseous moles are less on product sides. Hence ,increase in number of moles of products.
3.
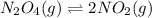
On increasing the pressure ,the equilibrium will shift in direction where decrease in pressure is taking place,so reaction will shift in backward direction because number of gaseous moles are less on reactant side both sides. Hence, decrease in number of moles of products.