The question is incomplete , complete question is :
Ammonium perchlorate
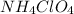 is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen gas, chlorine gas, oxygen gas and water vapor, releasing a great deal of energy. Calculate the moles of chlorine produced by the reaction of 2.5 mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.
is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen gas, chlorine gas, oxygen gas and water vapor, releasing a great deal of energy. Calculate the moles of chlorine produced by the reaction of 2.5 mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.
Answer:
1.2 moles of chlorine produced by the reaction 2.5 moles of ammonium perchlorate.
Step-by-step explanation:
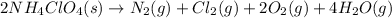
Moles of ammonium perchlorate = 2.5 moles
According to reaction, 2 moles of ammonium perchlorate on decomposition gives 1 mole of chlorine gas, then 2.5 moles of ammonium perchlorate wil give:
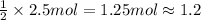 of chlorine gas
of chlorine gas
1.2 moles of chlorine produced by the reaction 2.5 moles of ammonium perchlorate.