Hii there !
The reaction accompanying production of water in this case would be -
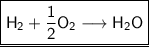
Thus ,by observing the reaction, we can conclude that 1 mole of Hydrogen (H
 )react with 1/2 mole of oxygen(O
)react with 1/2 mole of oxygen(O
 )to produce 1 mole of water (H
)to produce 1 mole of water (H
 O)
O)
 Therefore, 37.0 moles of hydrogen(H
Therefore, 37.0 moles of hydrogen(H
 ) will be required to produce 37.0 moles of water.
) will be required to produce 37.0 moles of water.
Hope it helps.