Answer : The value of work done for the system is, 29.7 J
Explanation :
First we have to calculate the moles of
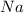
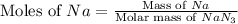
Molar mass of
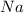 = 23 g/mole
= 23 g/mole
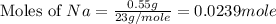
Now we have to calculate the volume of hydrogen.
As, 2 mole of Na produced 24.5 L volume of hydrogen gas
So, 0.0239 mole of Na produced
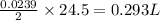 volume of hydrogen gas
volume of hydrogen gas
Now we have to calculate the magnitude of work done.
Work done = Pressure × Volume
Work done = 1.00 atm × 0.293 L
Work done = 0.293 L.atm
Conversion used : (1 L.atm = 101.3 J)
Work done = 0.293 × 101.3 = 29.7 J
Therefore, the value of work done for the system is, 29.7 J