Answer:
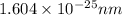 is the de Broglie wavelength associated with a baseball that is moving with a velocity of 42 mph.
is the de Broglie wavelength associated with a baseball that is moving with a velocity of 42 mph.
Step-by-step explanation:
De-Broglie's wavelength, which is:
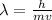
where,
 = De-Broglie's wavelength = ?
= De-Broglie's wavelength = ?
h = Planck's constant =
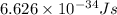
m = mass of particle =
v = velocity of the particle
We have :
Mass of baseball = m = 220 g = 0.220 kg ( 1g = 0.001 kg)
Velocity of the base ball = v = 42mph =
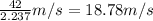
1 m/s = 2.237 mph
De-Broglie wavelength of the baseball at v:

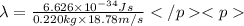 =1.604\times 10^{-34} m=1.604\times 10^{-25} nm[/tex]
=1.604\times 10^{-34} m=1.604\times 10^{-25} nm[/tex]
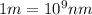
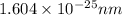 is the de Broglie wavelength associated with a baseball that is moving with a velocity of 42 mph.
is the de Broglie wavelength associated with a baseball that is moving with a velocity of 42 mph.