Answer:
a)= 0.025602u
b) = 23.848MeV
c) N = 1.546 × 10¹³
Step-by-step explanation:
The reaction is
²₁H + ²₁H ⇄ ⁴₂H + Q
a) The mass difference is
Δm = 2m(²₁H) - m (⁴₂H)
= 2(2.014102u) - 4.002602u
= 0.025602u
b) Use the Einstein mass energy relation ship
The enegy release is the mass difference times 931.5MeV/U
E = (0.025602) (931.5)
= 23.848MeV
c)
the number of reaction need per seconds is
N = Q/E
= 59W/ 23.848MeV
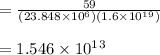
N = 1.546 × 10¹³