When calcium carbonate is heated, it produces calcium oxide and carbon dioxide. The equation for the reaction is CaCO3(s) CaO(s) CO2(g). How many grams of calcium carbonate (molar mass = 100 g/mol) need to decompose to produce 44.5 g of Calcium oxide.
Answer: 79.5 grams
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP, contains avogadro's number
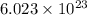 of particles and weighs equal to the molecular mass of the substance.
of particles and weighs equal to the molecular mass of the substance.
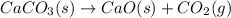
According to stoichiometry :
1 mole of CaO is produced by 1 mole of
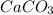
That means 56 g of CaO is produced by 100 g of
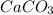
Thus 44.5 g of CaO is produced by =
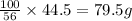 of
of
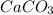
Thus 79.5 g of
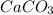 need to decompose to produce 44.5 g of Calcium oxide.
need to decompose to produce 44.5 g of Calcium oxide.