Answer:
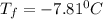
Step-by-step explanation:
Hello,
a) In this case, since the heat associated with the dissolution of ammonium nitrate is positive, such reaction is endothermic as it absorbs heat.
b) Now, for computing the temperature once the dissolution is done, we apply (considering that it is a cooling process):
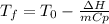
Nonetheless, we should first compute the moles of the mixture as:
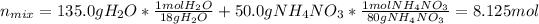
Thus, the total absorbed heat is:
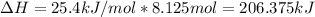
Now, the temperature is:
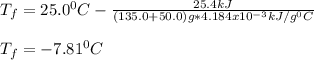
Best regards.