Answer:
Number of cases where internal energy of system decreases = 1
Step-by-step explanation:
According to first law of thermodynamics-
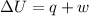
where,
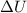 , q and w represents change in internal energy, heat exchanged and work done respectively.
, q and w represents change in internal energy, heat exchanged and work done respectively.
- When heat is absorbed (q > 0),
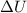 will also increase.
will also increase.
- When q = 30 J and w = 44 J,
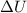 = (30+44) J = 74 J (positive). So, internal energy of system increases.
= (30+44) J = 74 J (positive). So, internal energy of system increases.
- When q = 0 and system does work on its surroundings (w < 0),
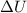 < 0 (negative). So, internal energy of system decreases.
< 0 (negative). So, internal energy of system decreases.
- When work id done on system (w > 0) and heat is withdrawn (q < 0) by an equal amount (w = -q),
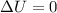 . So, internal energy does not change.
. So, internal energy does not change.
So, number of cases where internal energy of system decreases = 1