Answer:
The enthalpy change of the reaction is -66.88 kJ/mol.
Step-by-step explanation:
Mass of the solution = m = 100 g
Heat capacity of the solution = c = 4.18 J/g°C
Initial temperature of the solutions before mixing =
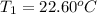
Final temperature of the solution after mixing =
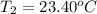
Heat gained by the solution due to heat released by reaction between HCl and silver nitrate = Q
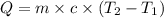
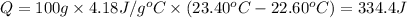
Heat released due to reaction = Q' =-Q = -334.4 J
Moles of silver nitrate = n
Molarity of silver nitrate solution = 0.100 M
Volume of the silver nitrate solution = 50.0 mL = 0.050 L ( 1 mL = 0.001 L)
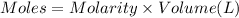
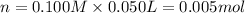
Enthalpy change of the reaction =

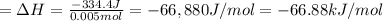
1 J = 0.001 kJ
The enthalpy change of the reaction is -66.88 kJ/mol.