Step-by-step explanation:
We know that relation between Gibb's free energy and temperature is as follows.
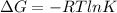
We are given that the value of
 is 1.7 kJ/mol.
is 1.7 kJ/mol.
And,
k =
![([product])/([substrate])](https://img.qammunity.org/2021/formulas/chemistry/college/9jvqadelvpxkafjdze0x585eiey9lboeoi.png)
=
![\frac{\text{[fructofuranose]}}{\text{[fructopyranose]}}](https://img.qammunity.org/2021/formulas/chemistry/college/1zfglul9d59z6x4um7r5plu0wjdtcou061.png)
Since, k = 0.50357 and temperature is equal to 298 K.
Therefore,
![\frac{\text{[fructofuranose]}}{\text{[fructopyranose]}}](https://img.qammunity.org/2021/formulas/chemistry/college/1zfglul9d59z6x4um7r5plu0wjdtcou061.png) = 0.50357
= 0.50357
so,
![\frac{\text{[fructofuranose]}}{\text{[fructopyranose]}}](https://img.qammunity.org/2021/formulas/chemistry/college/1zfglul9d59z6x4um7r5plu0wjdtcou061.png) + 1 = 1.50357
+ 1 = 1.50357
=
![\frac{\text{[total fructose solution]}}{\text{[fructopyranose]}}](https://img.qammunity.org/2021/formulas/chemistry/college/dh9be3j67r8joye8nm5lkjrb4ygknle4gk.png)
=
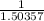
= 0.665
Therefore, we can conclude that 0.665 fraction of the total fructose in solution is fructopyranose.