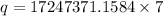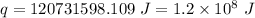Answer:
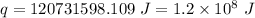
Step-by-step explanation:
Given:
time of shower usage each day,
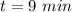
flow rate through the shower,
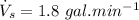
volume of water used for washing each day,
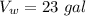
initial temperature of water,
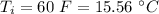
final temperature of water,
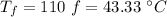
Now the total volume of water used each day:
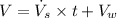
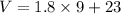
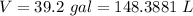
We know that the mass of 1 L of water is 1 kg, so the mass of water used each day:
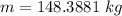
Heat energy required for heating the water each day within the given temperature range:
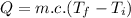
where:
c = specific heat capacity of water =
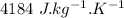
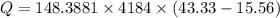
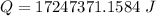
Now the energy required per week:
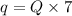
since there are 7 days in a week