Answer:
9.63 L.
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
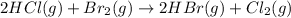
So the consumed amounts of hydrochloric acid and bromine are the same to the beginning based on:
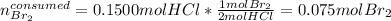
In such a way, the yielded moles of hydrobromic acid and chlorine are:
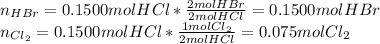
Thus, the volume of the sample, after the reaction is the same as no change in the total moles is evidenced, that is 9.63L.
Best regards.