Step-by-step explanation:
First, for 1.85 mol Co the enthalpy will be calculated as follows.
Enthalpy of vaporization,
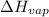 =
=
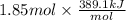
= 719.8 kJ
Now, we will convert the temperature into Kelvin as follows.
(3097 + 273) K
= 3370 K
Therefore, entropy of vaporization will be calculated as follows.
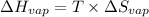
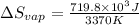
= 213.6 J/K
Thus, we can conclude that
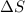 for vaporization is 213.6 J/K.
for vaporization is 213.6 J/K.