Answer: The partial pressure of
 if the total pressure of the mixture is 3.9 atm is 0.975 atm
if the total pressure of the mixture is 3.9 atm is 0.975 atm
Step-by-step explanation:
According to Raoult's law, the vapor pressure of a component at a given temperature is equal to the mole fraction of that component multiplied by the vapor pressure of that component in the pure state.
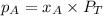
where, x = mole fraction of nitrogen in solution = 0.25
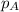 = partial pressure of nitrogen = ?
= partial pressure of nitrogen = ?
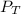 = Total pressure = 3.9 atm
= Total pressure = 3.9 atm
Putting in the values :
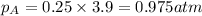
The partial pressure of
 is 0.975 atm
is 0.975 atm