Answer: There are 105 grams of acetylene in the tank.
Step-by-step explanation:
According to ideal gas equation:

P = pressure of gas = 1.39 atm
V = volume of gas = 70.0 L
n = number of moles = ?
R = gas constant =
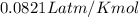
T = temperature of gas in Kelvin =
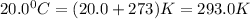
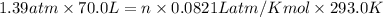
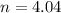
Mass of ethylene , M = Moles × Molar mass = 4.04 mole × 26 g/mol =105g
Thus there are 105 grams of acetylene in the tank.