Answer:
There are 4 tryptophans in the protein.
Step-by-step explanation:
According to question, protein contains one tyrosine residue and say x number of tryptophans.
Concentration of protein solution = 1.0 micromolar =
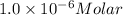
Molar absorptivity of a protein solution :


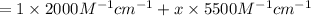
Length of the cuvette = l = 1.0 cm
Absorbance of protein solution at 280 nm = A = 0.024
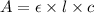 ( Beer-Lambert's law)
( Beer-Lambert's law)
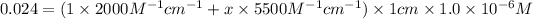
Solving for x :
x = 4
There are 4 tryptophans in the protein.