Step-by-step explanation:
(a). First of all, we calculate the number of atoms per cm^{3}, and by taking into account that each atom contributes with one free electron we have
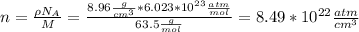
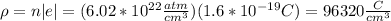
(b)
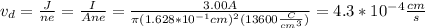
(c)
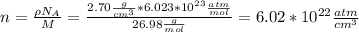
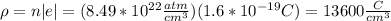
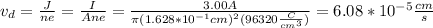
I hope this is useful for you
P.D please change 1.628 (the diameter) by 0.814(the radius) in the calculation
:)