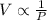Answer :
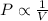 and
and
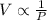
Step-by-step explanation:
According to Boyle's law, it states that pressure is directly proportional to the volume of the gas for a fixed amount of gas and at constant temperature.
The equation given by this law is:
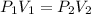
where,
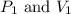 are initial pressure and volume.
are initial pressure and volume.
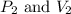 are final pressure and volume.
are final pressure and volume.
Thus the correct relation for pressure and volume for a fixed amount of gas at constant temperature according to Boyle’s law is
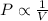 and
and