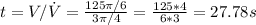Answer:
27.78 s
Step-by-step explanation:
Assume ideal gas, since pressure and temperature of the air inside the balloon is the same as air entering the balloon, then the volume (and its rate) stays the same.
The cross-sectional area of the hole where air enters
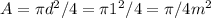
Hence the volume rate:
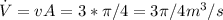
The total volume of the spherical balloon is
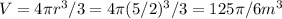
Hence the time it takes to fill this much at a constant rate of 3π/4 is