Answer:
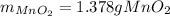
Step-by-step explanation:
Hello,
In this case, we first apply the ideal gas equation to compute the moles of produced chlorine:
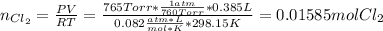
Then, by considering the given reaction, applying the stoichiometry, that shows a 1 to 1 relationship between chlorine and manganese dioxide, we find:
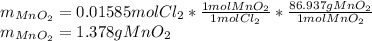
Best regards.