Answer:
a. C₆H₅OH(aq)
Step-by-step explanation:
The reaction of phenol with water is shown below as:-
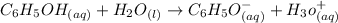
According to Arrhenius, The specie which furnish protons in solution is known as acid. In the above reaction, phenol,
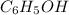 acts as an acid by contributing a H⁺ ion to create H₃O⁺ in the chemical equation.
acts as an acid by contributing a H⁺ ion to create H₃O⁺ in the chemical equation.
a. C₆H₅OH(aq) is the answer