Thus the value of [OH⁻] calculated is = 3.3 × 10⁻⁶ M
Step-by-step explanation:
Given:
Concentration of H₃O⁺ = 3.3 × 10⁻⁹ M
To Find:
Concentration of OH⁻ ion = ?
Solution:
From the
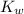 value, and the concentration of H⁺ ion, we can find the concentration of hydroxyl ions, as
value, and the concentration of H⁺ ion, we can find the concentration of hydroxyl ions, as
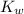 is the product of the concentration of H⁺ and OH⁻ ions. We have the concentration of hydronium ion or H⁺ ion and
is the product of the concentration of H⁺ and OH⁻ ions. We have the concentration of hydronium ion or H⁺ ion and
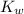 value, dividing
value, dividing
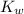 by the H⁺ ion concentration, we will get the concentration of OH⁻ ion as,
by the H⁺ ion concentration, we will get the concentration of OH⁻ ion as,
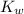 = [H₃O⁺] × [OH⁻]
= [H₃O⁺] × [OH⁻]
= 3.0 × 10⁻⁹ × [OH⁻]
Now we have to divide as,
[OH⁻] =
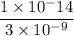 = 3.3 × 10⁻⁶ M
= 3.3 × 10⁻⁶ M