Answer:
32.5g
Step-by-step explanation:
Given parameters:
Mass of lithium hydroxide = 10g
Unknown:
Mass of lithium bromide produced = ?
Solution:
The equation of the reaction is shown below;
LiOH + HBr → LiBr + H₂O
This is an acid-base type of reaction called neutralization.
To solve this problem, work from the known specie to the unknown. The known specie here is LiOH.
Find the number of moles of LiOH;
Number of moles of LiOH =
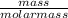
Molar mass of LiOH = 7 + 16 + 1 = 24g/mol
Number of moles =
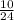 = 0.42moles
= 0.42moles
Now,
From the balanced chemical equation;
1 mole of LiOH produced 1 mole of LiBr
0.42 moles of LiOH will produce 0.42 moles of LiBr
To find the mass of LiBr produced;
Mass of LiBr = number of moles x molar mass
Molar mass of LiBr = 7 + 80 = 87g/mol
Mass of LiBr = 0.42 x 87 = 32.5g