The amount of Li present to start the reaction is 55.18g
Step-by-step explanation:
2Li + Br₂ → 2LiBr
Molecular weight of Br₂ = 159.808 g/mol
Mass of Br₂ present = 225 g
Moles of Br₂ present during the reaction = 225 / 159.808
m = 1.4
Molecular weight of LiBr = 86.845 g/mol
Mass of LiBr formed = 690 g
moles of LiBr produced = 690 / 86.845
m(LiBr) = 7.95
According to the balanced equation, 2 molecules of Li reacts to for 2 molecule of LiBr
So, 7.95 moles of LiBr would require 7.95 moles of Li
The molecular weight of Li is 6.941 g/mol
Thus, the amount of lithium present to start the reaction is
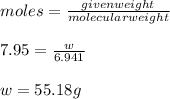
Therefore, the amount of Li present to start the reaction is 55.18g