Answer:
Step-by-step explanation:
The metal with the highest specific heat capacity will undergo the most temperature change.
Specific heat capacity is the amount of heat supplied to a unit mass of a substance to cause a temperature change of 1°C.
A very good conductor of heat will have low specific heat capacity. This implies that less heat will be required to cause a monumental change in its temperature.
C =
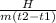
where C is specific heat
H is the amount of heat supplied
m is the mass
t is the temperature
We see that since both H and m for the two metals are the same, specific heat is inversely proportional to temperature change.
The lower the heat capacity, the higher the temperature change.