Answer:
0.73moles
128.48g
Step-by-step explanation:
Given parameters:
Mass of ICl₃ = 341g
Mass of H₂O = 151.7g
Unknown:
Maximum numbers of moles and grams of iodic acid = ?
Solution:
To solve this problem, one needs to obtain a balanced chemical equation for a start;
2ICl₃ + 3H₂O → ICI + HIO₃ + 5HCl
Now, we need to find the limiting reagent because it determines the extent of the reaction;
Number of moles =
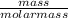
molar mass of ICl₃ = 127 + 3(35.5) = 233.5g/mol
molar mass of H₂O = 2(1) + 16 = 18g/mol
Number of moles of ICl₃ =
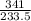 = 1.46moles
= 1.46moles
Number of moles of H₂O =
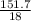 = 8.43moles
= 8.43moles
From the balanced equation;
2 moles of ICl₃ combined with 3 moles of water
1.46 moles of ICl₃ will need
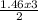 = 2.19 moles
= 2.19 moles
But we were given 8.43moles of water;
This suggests that water is excess
Now we can relate the known to the unknown:
2 moles of ICl₃ produced 1 mole of HIO₃
then 1.46 mole of ICl₃ will produce
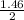 = 0.73moles
= 0.73moles
Mass of HIO₃ = number of moles x molar mass
= 0.73moles x (1 + 127 + 3(16))
= 128.48g