0.3 moles of lithium chloride (LiCI) reacted to produce 24.68 grams of lithium nitrate (LiNO3).
Step-by-step explanation:
Balanced equation for the reaction is:
2LiCl + Pb(NO3)2 ⇒2 LiNO3 + PbCl2
Data given:
mass of LiNO3 produced = 24.68 grams
moles of LiCl reacted= ?
atomic mass of 1 mole of LiNO3 = 68.946 Grams
Number of moles =
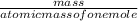
putting the values in the above equation:
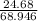
= 0.3 moles
from the reaction,
2 moles of LiCl reacted to form 2 moles of LiNO3
so, x moles of LiCl reacted to form 0.3 moles of LiNO3
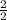 =
=
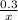
x = 0.3 moles of LiCl will react to form 0.3 moles of LiNO3.