Answer:
2.52L
Step-by-step explanation:
Given parameters:
T₁ = 400K
V₁ = 4L
T₂ = 252K
unknown
V₂ = ?
Solution:
To solve this problem, we are going to apply charle's law. The law states that the volume of a fixed mass of gas is directly proportional to temperature provided pressure is constant.
Mathematically,
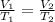
Substitute and solve for V₂
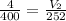
V₂ = 2.52L