Answer:
Step-by-step explanation:
Given parameters:
Mass of CuCl₂ = 10.5g
Mass of Aluminium = 12.49g
Unknown:
Limiting reactant = ?
Solution:
The limiting reactant is the reactant given in short supply. Such reactant determines and controls the extent of the reaction.
Once they are used up, the reaction ceases to progress.
To find such reactant, we need to have a balanced reaction equation and the number of moles of the reactants:
3CuCl₂ + 2Al → 3Cu + 2AlCl₃
number of moles =
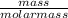
Molar mass of CuCl₂ = 63.5 + 2(35.5) = 134.5g/mol
Molar mass of Al = 27
Number of moles of CuCl₂ =
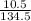 = 0.08moles
= 0.08moles
Number of moles of Al =
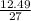 = 0.46moles
= 0.46moles
From the balanced reaction equation;
3 mole of CuCl₂ combined with 2 moles of Al
0.08 mole of CuCl₂ will combine with
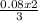 = 0.05moles of Al
= 0.05moles of Al
But the given amount of Al is 0.46moles which is in excess.
Therefore CuCl₂ is in the limiting reactant.