Joseph's percentage error would be 1.89%
Step-by-step explanation:
Exact value of the density of aluminium = 2.699 g/cm³
Calculated value of the density of aluminium = 2.75 g/cm³
Percentage error = ?
We know:
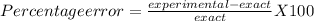
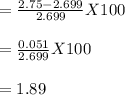
Therefore, Joseph's percentage error would be 1.89%