0.72 mole of oxygen would produce 320.4 kJ of heat.
Step-by-step explanation:
CH₄ (g) + 2O₂ (g) → CO₂ (g) + 2H₂O (ℓ) + 890kJ
According to the equation,
2 moles of O₂ produces 890 kJ of heat
So, 0.72 moles of O₂ will produce:
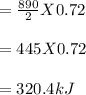
Therefore, 0.72 mole of oxygen would produce 320.4 kJ of heat.