Answer: The pH of the solution is 10.74
Step-by-step explanation:
- For methylammonium chloride:
To calculate the number of moles, we use the equation:

Given mass of methylammonium chloride = 0.405 g
Molar mass of methylammonium chloride = 67.52 g/mol
Putting values in above equation, we get:
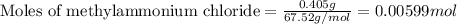
To calculate the number of moles for given molarity, we use the equation:
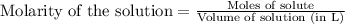
Molarity of methylamine solution = 0.300 M
Volume of solution = 25 mL = 0.025 L
Putting values in above equation, we get:

Total volume of the solution = [25 + 500] = 525 mL = 0.525 L (Conversion factor: 1 L = 1000 mL)
To calculate the pOH of basic buffer, we use the equation given by Henderson Hasselbalch:
![pOH=pK_b+\log(([salt])/([base]))](https://img.qammunity.org/2021/formulas/chemistry/college/whkb5aycrz2thv5lruk0dbi2nyi3z7t6qc.png)
![pOH=pK_b+\log(([CH_3NH_3^+Cl^-])/([CH_3NH_2]))](https://img.qammunity.org/2021/formulas/chemistry/college/qppceuk9c05imy0s7yh6sbrc68wvk3w1aw.png)
We are given:
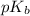 = negative logarithm of base dissociation constant of methylamine = 3.36
= negative logarithm of base dissociation constant of methylamine = 3.36
![[CH_3NH_2]=(0.0075)/(0.525)](https://img.qammunity.org/2021/formulas/chemistry/college/fr65xnr92hnlh6w5riy9t5qg2lkof6wbw6.png)
![[CH_3NH_3^+Cl^-]=(0.00599)/(0.525)](https://img.qammunity.org/2021/formulas/chemistry/college/d2xyapwhfyc4s3eqld7kgdjib0p9gduniy.png)
pOH = ?
Putting values in above equation, we get:
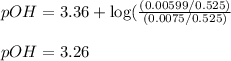
To calculate the pH of the solution, we use the equation:
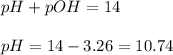
Hence, the pH of the solution is 10.74