Answer:
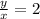
Step-by-step explanation:
Hello,
In this case, since the subscripts for both carbon and oxygen are equal, one could assume the following empirical formula:
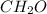
Which is suitable for formaldehyde or if it is multiplied by 2 (C₂H₄O₂) for acetic acid or ethen-1,2-diol. In such a way, computing the percent composition:
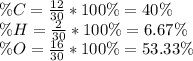
Thus, if at the beginning we just have, the percentages, one could compute moles as:
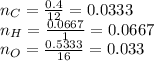
Whereas based on the initial formula:
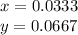
The ratio
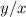 will be:
will be:
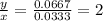
For all the whole numbers the empirical formula was multiplied by.
Best regards.