Answer:
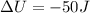
Step-by-step explanation:
Hello,
In this case, we use the equation regarding the first law of thermodynamics which is:
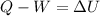
In this case, the want to compute the change in thermal energy of the gas which is related with ΔU. In such a way, since the 10 J are being removed, they are set as negative in the aforesaid equation, moreover, the 40 J of work are positive since it is a work done on the system. Hence:
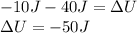
Best regards.