Answer:
The balanced chemical reaction is given as:
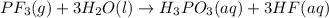
b) Concentration of phosphorus acid is 0.0803 M.
Concentration of hydrofluoric acid is 0.241 M.
Step-by-step explanation:
a) The balanced chemical reaction is given as:
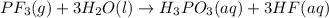
b)
Moles of phosphorus trifluoride gas =

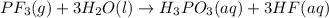
According to reaction, 1 mole of phosphorus trifluoride gives 1 mole of phosphorus acid, then
 of phosphorus trifluoride will give:
of phosphorus trifluoride will give:
 of phosphorus acid
of phosphorus acid
Volume of the solution = V = 711 mL = 0.711 L ( 1 mL = 0.001 L)
![[concentration]=(Moles)/(Volume(L))](https://img.qammunity.org/2021/formulas/chemistry/college/uoiisieojnqyxankh5y780bpfhhb48jaoh.png)
Concentration of phosphorus acid :
![[H_3PO_3]=(5.71* 10^(-2) mol)/(0.711 L)=0.0803 M](https://img.qammunity.org/2021/formulas/chemistry/college/forixg22kdbereesys6e25nh3x08ocj5nc.png)
Concentration of phosphorus acid is 0.0803 M.
According to reaction, 1 mole of phosphorus trifluoride gives 3 mole of hydrofluoric acid, then
 of phosphorus trifluoride will give:
of phosphorus trifluoride will give:
 of hydrofluoric acid
of hydrofluoric acid
Volume of the solution = V = 711 mL = 0.711 L ( 1 mL = 0.001 L)
![[concentration]=(Moles)/(Volume(L))](https://img.qammunity.org/2021/formulas/chemistry/college/uoiisieojnqyxankh5y780bpfhhb48jaoh.png)
Concentration of hydrofluoric acid :
![[HF]=(0.1713 mol)/(0.711 L)=0.241 M](https://img.qammunity.org/2021/formulas/chemistry/college/g7wuy0jivftidq8cgp81c0qy58isiy0a2q.png)
Concentration of hydrofluoric acid is 0.241 M.