Options are not given, however, the following reactions occurs when Group A ions are reacted with HCl followed by NH3
Answer:
Gray precipitate is seen, which confirms the presence of mercury ions
Step-by-step explanation:
Selective precipitation is a qualitative analysis, which involves addition of a carefully selected reagents to an aqueous mixture of ions. This results in the precipitation of one or more ions, while leaving the rest in solution. Later, a reaction specific to that ion is carried out separately to determine its identity.
HCl react with both Ag+ and Hg+ ions to form the following precipitates:
Ag+(aq) + Cl-(aq) → AgCl(s)
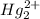 (aq) + 2Cl- →
(aq) + 2Cl- →
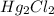 (s)
(s)
The precipitate, i.e silver chloride and mercury(I) chloride is removed and solution of NH3 is added.
Silver chloride will dissolve since its forms a soluble complex ion:
AgCl(s) +
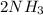 (aq) →
(aq) →
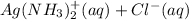
However, Mercury(I) chloride will react with ammonia to form a gray solid which is actually a mixture of black mercury and white
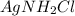 :
:
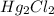 (s) +
(s) +
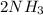 (aq) → Hg(l) +
(aq) → Hg(l) +
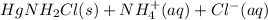
The presence of gray solid is the confirmation of the presence of
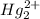 ion
ion