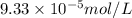This is an incomplete question, here is a complete question.
Calculate the solubility of each of the following compounds in moles per liter. Ignore any acid-base properties.
CaCO₃, Ksp = 8.7 × 10⁻⁹
Answer : The solubility of CaCO₃ is,
Explanation :
As we know that CaCO₃ dissociates to give
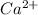 ion and
ion and
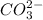 ion.
ion.
The solubility equilibrium reaction will be:
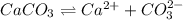
The expression for solubility constant for this reaction will be,
![K_(sp)=[Ca^(2+)][CO_3^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/xyd5s3ein7jqgamaxt1hn0c20i8rq5fuv0.png)
Let solubility of CaCO₃ be, 's'
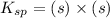
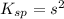
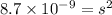
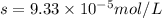
Therefore, the solubility of CaCO₃ is,