Answer:
The percent yield is about 66.6%, but with sig figs it's 70%
Step-by-step explanation:
The formula for percent yield is: (Actual Yield/Theoretical Yield) * 100
1. In order to solve for percent yield, you need both an actual and theoretical yield. This problem gives us an actual yield for H2O, which is 75 grams. Great, you're halfway there!
2. Next, you need to solve for the theoretical yield. In order to do so, you need to set up your stoichiometric ratio.
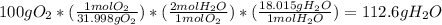
a) Take your given 100g of O2 and first convert it to moles
b) Then take the O2 in moles and create your mole-to-mole ratio. You're only given 2 values (75g of H2O and 100g of O2), so you know to use O2 and H2O in your ratio. The coefficients are essentially your ratio, so for H2O to O2 it's 2:1
c) Then convert the value from the mole-to-mole ratio back into grams to get your theoretical yield (as values from mole ratios are in moles)
3. Now that you have both your actual and theoretical yields, you can plug it into the equation and solve.
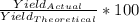 =
=
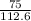 = 66.60403646
= 66.60403646
Which is 66.6% when rounded. With sig figs, again, it is 70%