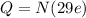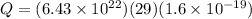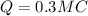Answer:
total positive charge in one copper penny is 0.3 MC
Step-by-step explanation:
As we know that the atomic number of copper is
z = 29
now moles of copper present in one copper penny is given as
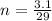
now total number of copper atoms present in it
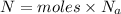
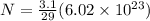
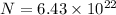
Now we know that in one atom there exist 29 protons and 29 electrons
so total positive charge in it