Answer:
The vapor pressure of the 70% isopropanol solution is 21 Torr.
Step-by-step explanation:
Relative lowering of vapor pressure for solution with non volatile solute is given by :
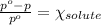
Where:
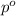 = Vapor pressure of pure solvent
= Vapor pressure of pure solvent
p = Vapor pressure of solution
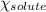 = Mole fraction of solute
= Mole fraction of solute
We have, 70% isopropanol solution which menas 70 grams of isopropanol (solvent) and 30 grams of water (solute).
Mass of water = 30 g
Moles of water =
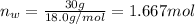
Mass of isopropanol = 70 g
Moles of isopropanol =
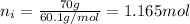
Mole fraction of solute that is water :
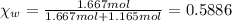
Vapor pressure of the pure isopropanol =
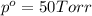
Vapor pressure of the solution = p
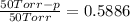
Solving for p:
p = 20.57 Torr ≈ 21 Torr
The vapor pressure of the 70% isopropanol solution is 21 Torr.