Answer: The pH of the solution is 7.52
Step-by-step explanation:
To calculate the pOH of the solution, we use the equation:
![pOH=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/aptpm2b2equoweomw80psbpn50765hcb2n.png)
We are given:
![[OH^-]=3.31* 10^(-7)](https://img.qammunity.org/2021/formulas/chemistry/college/vis5cpvg85cf05cekj7qvc5mzchwe3efdg.png)
Putting values in above equation, we get:
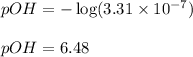
To calculate pH of the solution, we use the equation:
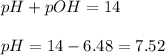
Hence, the pH of the solution is 7.52