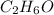Answer:
Step-by-step explanation:
The first step is the calculation of the moles of
 and
and
 , so:
, so:
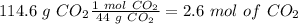
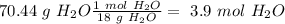
Now, in 1 mol of CO2 we have 1 mol of C and in 1 mol of
 we have 1 mol of H. Additionally, if we want to calculate the moles of oxygen we need to calculate the grams of C and O and then do the substraction form the initial amount, so:
we have 1 mol of H. Additionally, if we want to calculate the moles of oxygen we need to calculate the grams of C and O and then do the substraction form the initial amount, so:
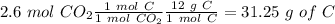
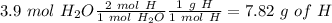
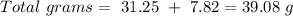

Now we can convert the grams of O to moles, so:
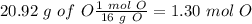
The next step is to divide all the mol values by the smallest one:
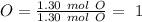
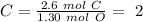
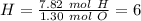
Therefore the formula is