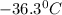Answer: Temperature of one liter of phosphine gas with a mass of 1.725 g and gas pressure of 0.9910 atm is
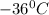
Step-by-step explanation:
According to the ideal gas equation:
PV=nRT
P = Pressure of the gas = 0.9910 atm
V= Volume of the gas= 1.0 L
T= Temperature of the gas in kelvin = ?
R= Gas constant =
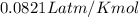
n= moles of gas=
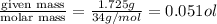
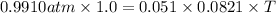
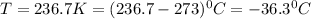
Thus the temperature (in °C) of one liter of phosphine gas with a mass of 1.725 g and gas pressure of 0.9910 atm is