Answer: The standard entropy of vaporization of ethanol is 0.275 J/K
Step-by-step explanation:
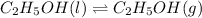
Using Gibbs Helmholtz equation:
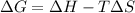
For a phase change, the reaction remains in equilibrium, thus
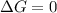
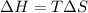
Given: Temperature = 285.0 K
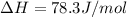
Putting the values in the equation:
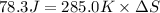
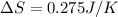
Thus the standard entropy of vaporization of ethanol is 0.275 J/K