Answer:
Kp = 0.35 & Kc = 0.0075
Step-by-step explanation:
PCl₅ ⇄ PCl₃ + Cl₂
Initial(atm) 0.5 0 0
At equilibrium 0.5 - p p p
- Total pressure at equilibrium = 0.78
- 0.5 - p + p + p = 0.78
- p = 0.28
So partial pressure of PCl₅ = 0.5 - 0.28 = 0.22 atm
Similarly PCl₃ = 0.28 atm & Cl₂ = 0.28 atm
According to law of mass action
- Kp =
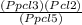
- Kp =
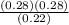
- Kp = 0.35
We know Kp = Kc x (RT)Δng
Δng = change in gaseous moles
= product moles - reactant moles
= 2 - 1
= 1
R = universal gas constant = 0.0821 lit. atm. mol⁻¹. K⁻¹
So Kc =
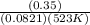
= 0.0081