Answer:
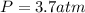
Step-by-step explanation:
Hello,
In this case, it is possible to determine the pressures of both helium and neon as shown below:
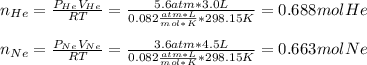
Now, one considers the total moles (addition between both neon's and helium's moles) and the total volume to compute the final pressure as shown below:
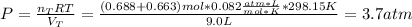
Best regards.