Answer:
Oxidation half-reaction.
Step-by-step explanation:
In electrolytic cell the anode is the positive electrode and the reaction occurring at anode is called Oxidation half-reaction.
The reaction is
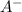 ⇒
⇒
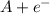
In a half-reaction there is a change in the oxidation state of the individual species involved in the reaction. In the above oxidation half-reaction the -1 oxidation state of A changes to 0 when it loses an electron. And as per the definition oxidation is said to occur when there is a loss of one or more electrons.